
Hacking Hospital Equipment is Child's Play
- By Ginger Hill
- Apr 29, 2014
When a security consultancy performed a penetration test on an Essentia Health network, the discovery was shocking, prompting a full-on investigation led by Scott Erven, head of information security for Essentia Health, and his team.
Over the course of two years, Erven’s team discovered:
- Drug infusion pumps for delivering morphine drips, chemo and antibiotics could be remotely manipulated to change the dosage given to patients;
- Bluetooth-enabled defibrillators could be manipulated to deliver random shocks or none to a patient’s heart;
- X-rays could be accessed by network lurkers;
- Temperature settings on blood and drug storing refrigerators could be reset;
- Digital medical records could be altered, causing misdiagnosis; and
- Some devices could be blue-screened, restarted or rebooted by hackers, wiping out configuration settings.
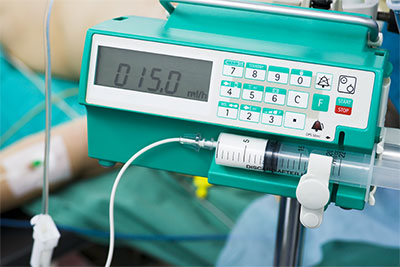 Specific brands of equipment have yet to be released, but Erven did notice some common security holes across the majority of the devices including lack of authentication to access equipment; weak, default and hardcoded vendor passwords being used; and embedded web servers, making it easy to manipulate devices when found on the network.
Specific brands of equipment have yet to be released, but Erven did notice some common security holes across the majority of the devices including lack of authentication to access equipment; weak, default and hardcoded vendor passwords being used; and embedded web servers, making it easy to manipulate devices when found on the network.
It was unknown if any of the devices tested by the team were connected to the Internet; however, many are connected to internal networks accessible via the Internet. This could allow hackers to gain access to devices by infecting employee computers with a phishing attack. Once inside the system, hackers can explore internal networks to find vulnerable systems. A hacker could even simply come into the hospital facility and plug his laptop into the network, allowing discovery and attacks on vulnerable systems.
Even though this is just a single study performed on one hospital network, the health care industry as a whole is lacking when it comes to security issues with medical equipment. This is probably because medical equipment has only been regulated for reliability, effectiveness and safety…not security.
According to Erven, vendors must do more to secure devices with encryption and authentication before equipment is sold to customers and if their devices are already in the field, vendors should fix them.
Sometimes vendors tell customers that hardcoded passwords can’t be removed from their equipment because it would require them to take the systems back to the FDA for approval after passwords are changed, but this is simply not true. FDA guidelines for medical equipment include a cybersecurity clause, allowing devices to be patched without recertification. These guidelines also say that vendors need to ensure that their systems are secure and patched, and customers should demand this.
About the Author
Ginger Hill is Group Social Media Manager.