SIA Submits Letter to FDA Addressing Thermal Imaging Systems
SIA recently submitted a letter to the Food and Drug Administration (FDA) Commissioner Stephen Hahn with a focus on an awareness that some companies are not following FDA guidelines related to the use of thermal imaging systems during the COVID-19 pandemic. The letter also encouraged the enforcement of FDA’s guidelines. The letter is available here.
SIA developed and issued the letter in October with input from an ad hoc group of SIA members who responded to a call for volunteers; the ad hoc group represented established producers and resellers of thermal camera systems in addition to other security industry companies which had a business or general interest in the technology.
As captured through this group and from direct feedback from members, it was clear many SIA members were concerned with thermographic camera systems for a variety of reasons. Some of these concerns that were voiced included:
Many companies have spent extensive resources on research and development testing their solutions against standards so that they can be used as a medical device. This included third-party testing and or the FDA’s 510(k) clearance process. Companies were concerned that tested products were being undercut by untested (and thus less expensive) products.
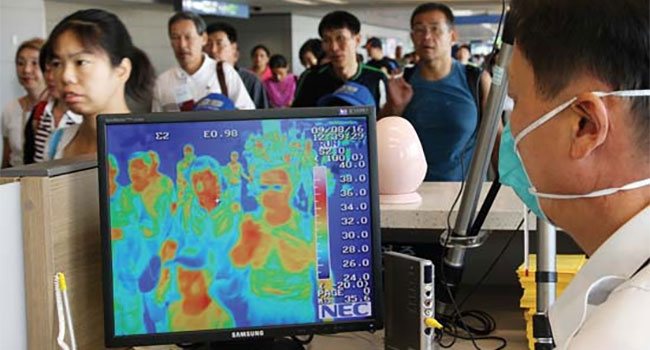
There were media reports of a number of products that did not work as marketed – particularly claims related to detecting temperatures of multiple subjects at a time, including while those multiple subjects were in motion, and claims that solutions were able to return accurate body/surface temperatures when the procerus region (the area around the bridge of the nose and corners of the eyes) was covered.
There were also reports of vendors and resellers claiming that their thermographic camera solutions are not medical devices and are therefore not subject to FDA guidelines. The group noted that there appears to be confusion as to what constitutes a medical device. The FDA’s definition of a medical device includes thermographic cameras that screen for temperature – or plainly any device that is “intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals” [emphasis added by SIA].
The input from members was that there are products being installed with the intention of disease management and mitigation, and that management and mitigation ultimately do not occur – because some of the products are unable to work as advertised, and this then leads to a false sense of safety.
“SIA believes it is extremely important that these thermographic solutions are marketed appropriately and implemented in line with the FDA’s guidelines,” said SIA CEO Don Erickson. “Circumventing the implementation guidelines can lead to market confusion and create a false sense of public safety related to the mitigation of the COVID-19 pandemic.”
The ad hoc group of SIA members also plans to follow up with additional guidance to the industry and the public that aligns with the FDA’s guidelines.